High Diagnostic Accuracy (~0.92 AUC) in Differentiating Viral from Non-Viral Infections — Explore Zybio’s POCT MxA Kit for Better ARI Management
April 16, 2025 Zybio News
-

December 26, 2025
DiagHub Online Academic Platform The ninth session on Microbiology Concludes Successfully – Highlights Recap
-

Nov 28, 2025
DiagHub Online Academic Platform The eighth session on Molecular Concludes Successfully – Highlights Recap
-

Nov 21, 2025
Zybio at MEDICA 2025: Comprehensive Laboratory Diagnostic Solution
In ESCMID 2025, Zybio had 4 posters successfully accepted, which were showcased in ESCMID hall during the congress. One of them, titled “Diagnostic Accuracy of MxA Protein as a Biomarker for Acute Viral Infections: A Multi-Center Validation Study”, evaluates Zybio’s POCT MxA kit in differentiating viral infection from non-viral infection, and the research shows ~0.92 AUC. This study supports the use of MxA as a reliable biomarker in the rapid diagnosis of acute respiratory infections (ARIs), facilitating more targeted clinical decisions.

Clinical Challenges in ARI Treatment: Why Rapid Diagnostics Matter More Than Ever
Acute respiratory infections (ARIs) are among the most common infectious diseases worldwide¹, yet distinguishing between bacterial and viral causes remains a clinical challenge—especially given the non-specific symptoms and limited availability of fast, accurate diagnostics². This often leads to overuse of antibiotics, exacerbating the global issue of antimicrobial resistance (AMR).
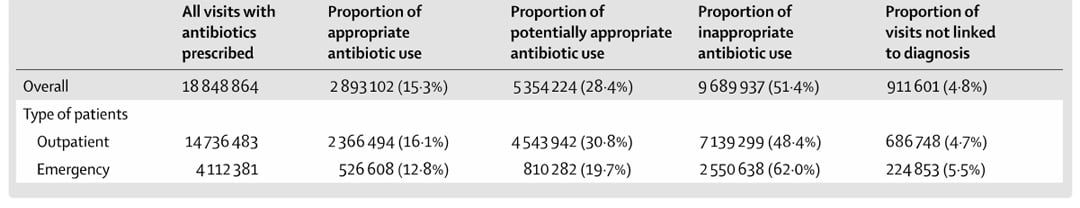
A nationwide study published in The Lancet analyzing over 170 million outpatient visits across China revealed that more than 50% of prescribed antibiotics were deemed possibly inappropriate, further highlighting the urgent need for more targeted and timely diagnostics.
Why MxA?
Unlike traditional inflammatory markers such as CRP, SAA or PCT, myxovirus resistance protein A (MxA) is highly specific to viral infections. MxA is an effector protein induced by type I (IFN-α/β) and type III (IFN-λ) interferons, and it belongs to the family of interferon-stimulated gene factors (ISGFs). MxA possesses broad-spectrum antiviral activity and plays a pivotal role in the innate antiviral immune response across nearly all vertebrates, responding rapidly to a wide variety of RNA and DNA viruses. Upon interferon stimulation, MxA levels rise within 1–2 hours, peak at approximately 16 hours, and have a half-life of 2.3 days. Its expression is tightly regulated by IFN-α/β and IFN-λ signaling and demonstrates a dose-dependent correlation with interferon levels. Importantly, MxA is not directly triggered by viral particles or other external stimuli, making its concentration in blood a highly reliable and specific biomarker for identifying acute viral infections.
Study Highlights: MxA Achieves ~0.92 AUC in Differentiating ARI
This multi-center clinical validation, conducted across three leading hospitals in China, evaluated Zybio's innovative MxA point-of-care testing (POCT) kit—making the first commercially available MxA diagnostic product developed in China. The study demonstrated MxA’s AUC of about 0.92 in distinguishing viral from non-viral acute respiratory infections.
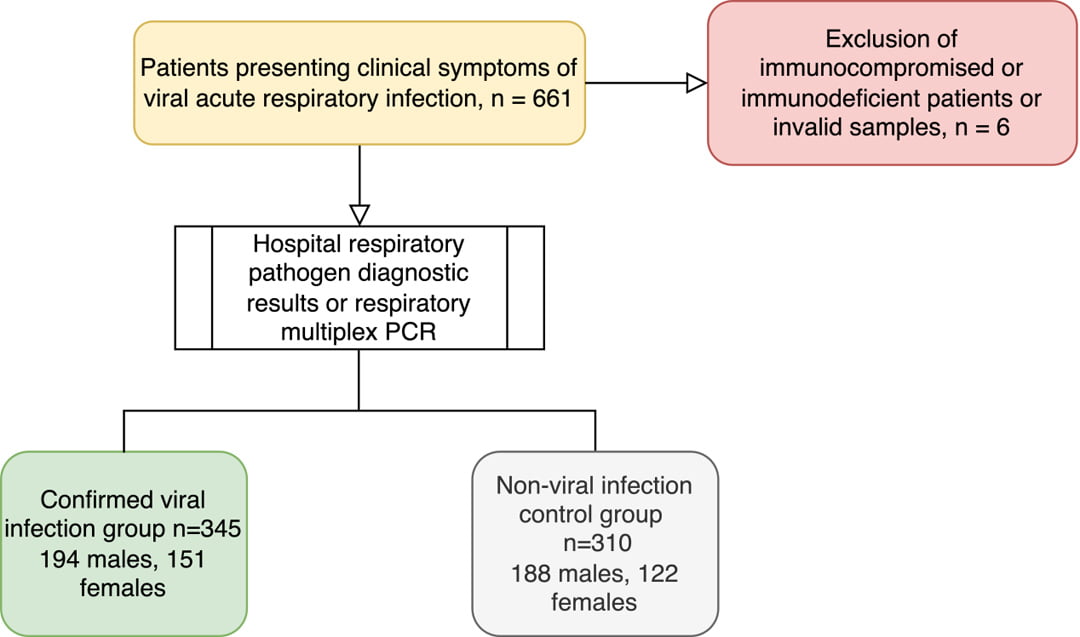
Figure 1: flow diagram for study enrollment
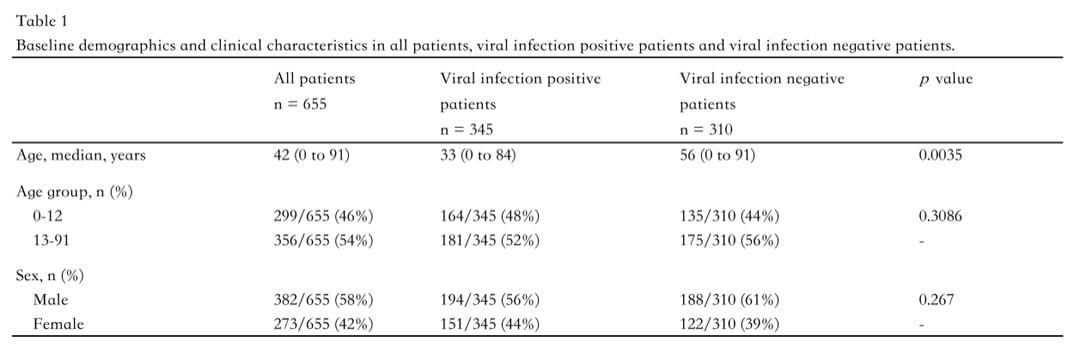
In total, 655 whole blood samples were analyzed—345 from the viral infection group (194 males, 151 females) and 310 from the control group (188 males, 122 females). Diagnostic performance was evaluated using receiver operating characteristic (ROC) curve analysis, with metrics including the area under the curve (AUC), sensitivity, and specificity.
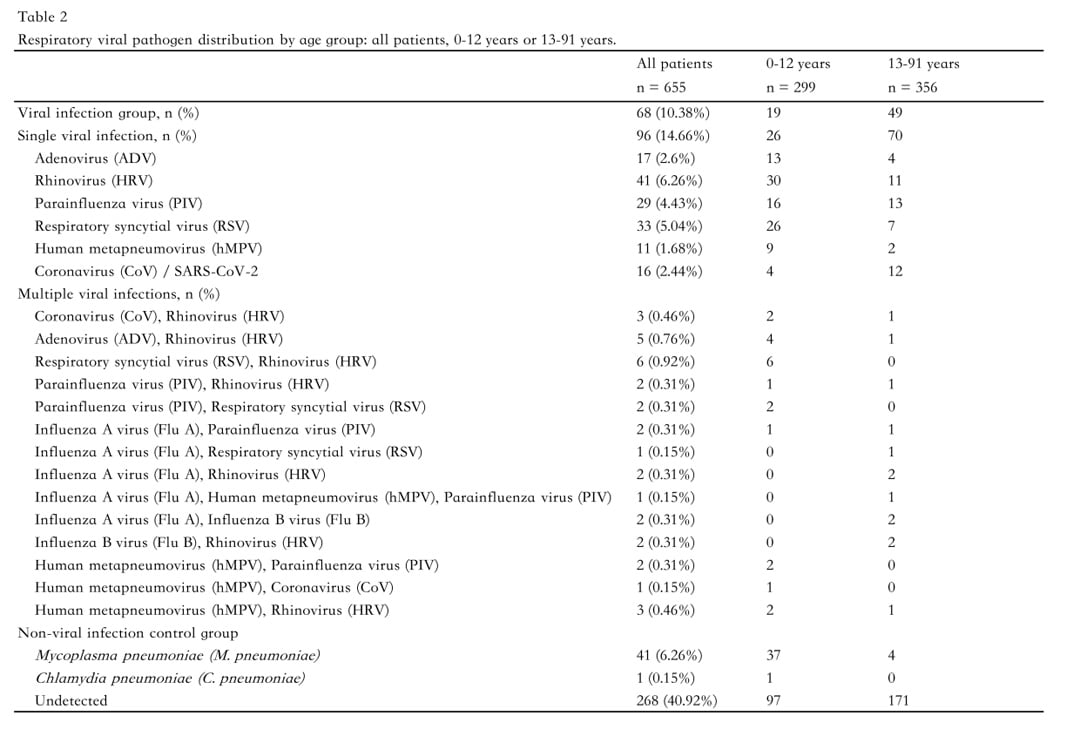
Among the 345 individuals in the confirmed viral infection group, single-virus infections accounted for the majority (85%), with the most frequently identified pathogens being human rhinovirus (HRV, 29%), respiratory syncytial virus (RSV, 20%), and influenza A virus (Flu A, 14%). The remaining 15% of this group had multiple viral infections.
In the non-viral infection control group (n=310), Mycoplasma pneumoniae was the most commonly detected pathogen, accounting for 6.26% of cases. In addition, no respiratory pathogen was detected in 268 participants (40.92%).
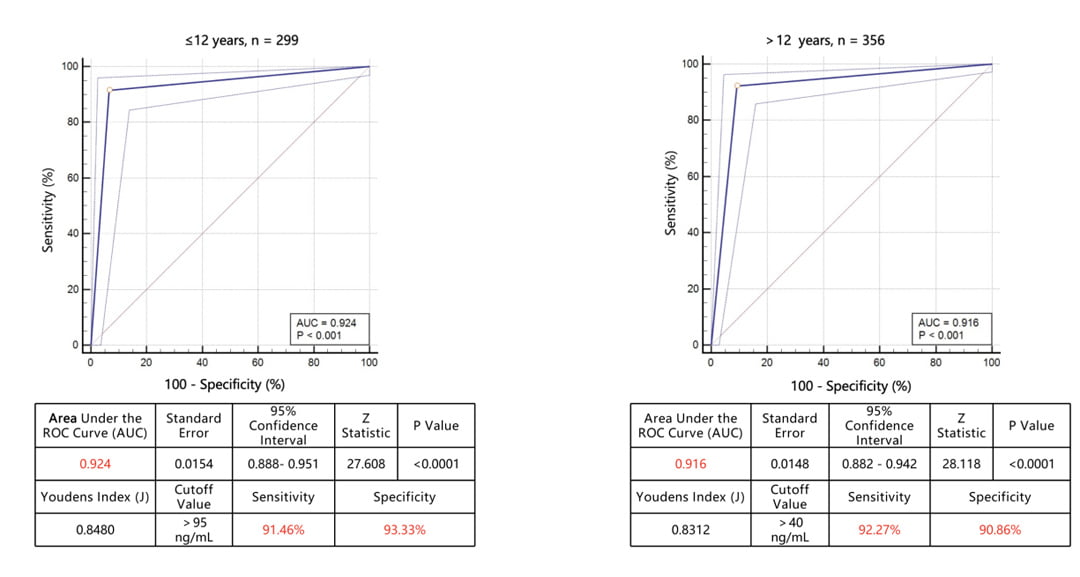
Figure 2: ROC curve of sensitivity versus specificity for the MxA test. (A) ROC and AUC for the MxA test for patients under 12 years old (n=299). (B) ROC and AUC for the MxA test for patients beyond 12 years old (n=356)
In patients aged ≤12 years (n = 299), MxA showed an AUC of 0.924 (95% CI: 0.888–0.951; p < 0.0001), with an optimal threshold of >95 ng/mL, yielding a sensitivity of 91.46% and a specificity of 93.33%. In aged over 12 years (n = 356), MxA performed similarly well, with an AUC of 0.916 (95% CI: 0.882–0.942; p < 0.0001), an optimal threshold of >40 ng/mL, sensitivity of 92.27%, and specificity of 90.86%. These results confirm that MxA delivers consistently high diagnostic accuracy across both pediatric and adult populations.
Zybio POCT MxA: Facilitating Precision Medicine for Infections and Combating AMR
This multi-center study demonstrated that the MxA point-of-care testing (POCT) kit features excellent diagnostic accuracy for identifying viral infections in both pediatric and adult patients. With its high sensitivity and specificity, it offers a reliable and efficient solution for distinguishing viral from non-viral infections—empowering more precise clinical decisions in respiratory infection management and supporting global efforts to combat antimicrobial resistance (AMR).
Zybio has been and will always be committed to the concept of "Dedication to Accuracy" and constantly go further in technology of laboratory testing. Meanwhile, we, with customer-centered notion in mind, will improve our services in an all-round and multi-angle manner to provide more comprehensive and efficient solutions for healthcare providers, and in this way, we safeguard patients' lives and health.
References
1. Li ZJ, Zhang HY, Ren LL, et al. Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. 2021;12(1):5026. doi:10.1038/s41467-021-25120-6
2. Zaas AK, Garner BH, Tsalik EL, Burke T, Woods CW, Ginsburg GS. The current epidemiology and clinical decisions surrounding acute respiratory infections. Trends Mol Med. 2014;20(10):579-588. doi:10.1016/j.molmed.2014.08.001