EU CE Certification | New COVID-19 detection products
January 15, 2021 Zybio News
-

Jun 27, 2025
DiagHub Online Academic Platform: The third session on POCT Concludes Successfully – Highlights Recap
-

May 30, 2025
DiagHub Online Academic Platform: The second session on Immunology Concludes Successfully
-

May 28, 2025
Conference Summary | 3rd Conference on Innovative Technology Applications in Laboratory Medicine: Exploring the Source of Mass Spectrometry, Advancing the Path of Innovation
Recently, Zybio's SARS-CoV-2 & Influenza A/B Nucleic Acid Detection Kit (PCR-Fluorescent Probe Method), SARS-CoV-2 Antigen Assay Kit (Colloidal Gold Method) obtained the CE certification.
Up to now, Zybio is able to provide a variety of COVID-19 related products for the world, including automatic nucleic acid extraction system EXM6000, EXM3000, EXM9600, nucleic acid extraction kit, SARS-CoV-2 Nucleic Acid Detection Kit, Molaccu SARS-CoV-2 B.1.1.7 Variant Assay Kit, SARS-CoV-2 & Influenza A_B Nucleic Acid Detection Kit, SARS-CoV-2 Antigen Assay Kit, SARS-CoV-2 IgM_IgG Antibody Assay Kit and other products, which provides a more comprehensive solutions to help to fight against COVID-19.
CE Marked Products
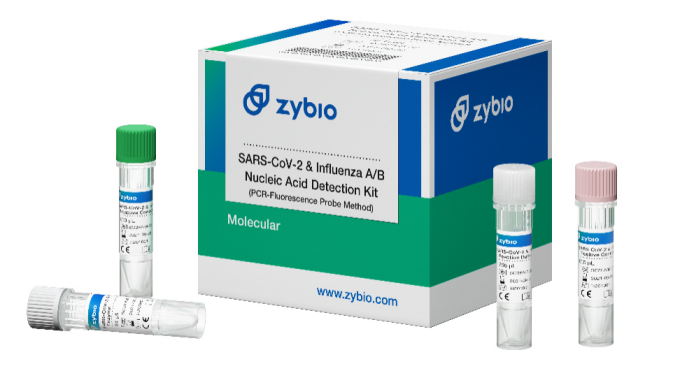
SARS-CoV-2 & Influenza A/B Nucleic Acid Detection Kit (PCR-Fluorescence Probe Method)
Early winter and spring are the seasons for influenza A and influenza B. COVID-19 pandemic is still developing this year, and its infection symptoms are similar to of influenza A and influenza B. This poses a new test for the prevention and control of current pandemic, so the differential diagnosis of the viruses is particularly important. SARS-CoV-2 & Influenza A/B Nucleic Acid Detection Kit can identify the type of virus through the detection of nasal/throat swab specimens, so as to achieve the goal of precise prevention and control of COVID-19 pandemic.
SARS-CoV-2 Antigen Assay Kit (Colloidal Gold Method)
In the context of the global COVID-19 pandemic, rapid test has become an important option, especially in countries with insufficient nucleic acid detection capabilities. When faced with a large number of test needs, SARS-CoV-2 Antigen Assay Kit as an auxiliary diagnosis has become important supplement to fight against the pandemic prevention and control.

CE certification
"CE" is read as an abbreviation of "conformité européenne". In the EU market, the "CE" mark is a compulsory certification mark. The product can be freely circulated in the EU market only after obtaining the "CE" certification, indicating that the product complies with the requirements of the EU Medical Device Directive. At present, these two products have been registered with the competent authorities of the European Union and have the access conditions for the European Union market.
Up to now, ZYbio has provided COVID-19 related products to nearly 90 countries around the world, covering Asia, South America, North America, Europe and Africa, helping global epidemic prevention and control.