Zybio hs-cTnI: A Heartfelt Commitment to Accuracy
August 15, 2024 Zybio News
-

September 26, 2025
DiagHub Online Academic Platform The sixth session on Hematology Concludes Successfully – Highlights Recap
-

August 29, 2025
DiagHub Online Academic Platform The fifth session on Microbiology Concludes Successfully – Highlights Recap
-

August 22, 2025
Zybio hs-cTnI Assay Achieves IFCC C-CB Listing: Global Witness for Excellence in Cardiac Diagnostics
Recently, Zybio’s hs-cTnI performance evaluation is successfully submitted to ADLM and the poster was displayed at the Expo. The study enrolled over 1200 patients from the Third Affiliated Hospital of Chongqing Medical University (a large tertiary hospital) and validated the excellent analytical performance of Zybio hs-cTnI assays.

Improved Antibody Engineering for Lower Limit of Detection
Zybio’s hs-cTnI reagent adopts three optimized antibodies, which bind to the sites in the most stable region of Troponin I (aar 34-126). This design mitigates the impact of various degradation fragments of cardiac troponin I released into circulation, allowing for the detection of lower concentrations of cardiac troponin. Consequently, the assay exhibits significantly improved sensitivity, enabling earlier detection of acute coronary syndrome (ACS). Additionally, the lower limit of detection facilitates rapid and accurate ruling out of myocardial infarction in clinical settings, thereby enhancing the negative predictive value (NPV).
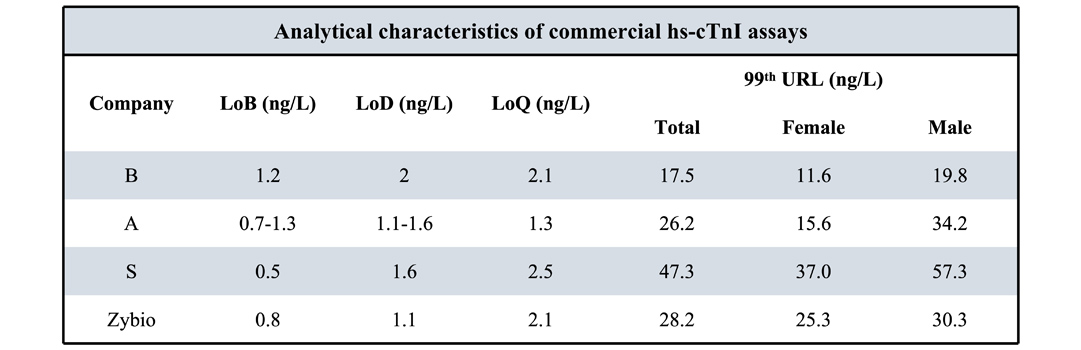
Data comparison between Zybio hs-cTnI and three major international manufacturers
*Data from Chinese Expert Consensus on Cardiac Troponin Laboratory Tests and Clinical Applications and manufacturer's Instructions for Use
Improved Technology for Excellent Detection Consistency
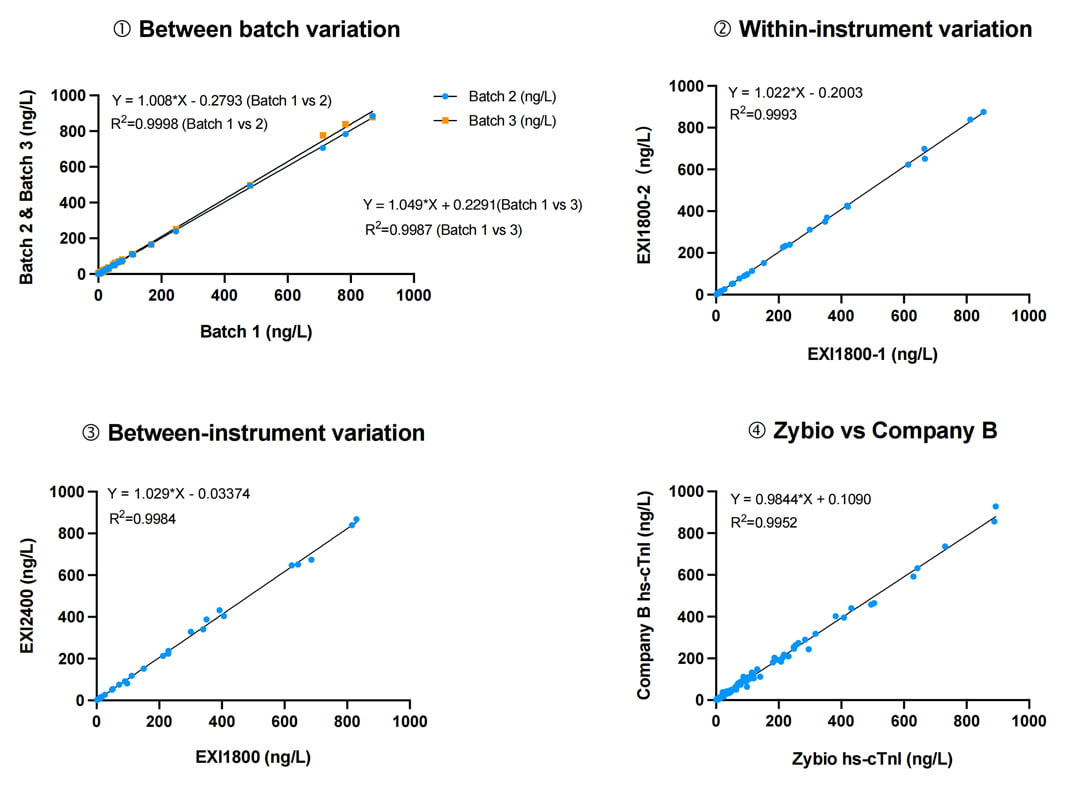
A comprehensive comparative evaluation was conducted to assess the product validation across different batches (① Between-batch variation), between instruments of the same model (② Within-instrument variation), between instruments of different models (③ Between-instrument variation), and against the international leading brand B (④Zybio vs Company B). The results show the high stability and consistency of Zybio's reagents.
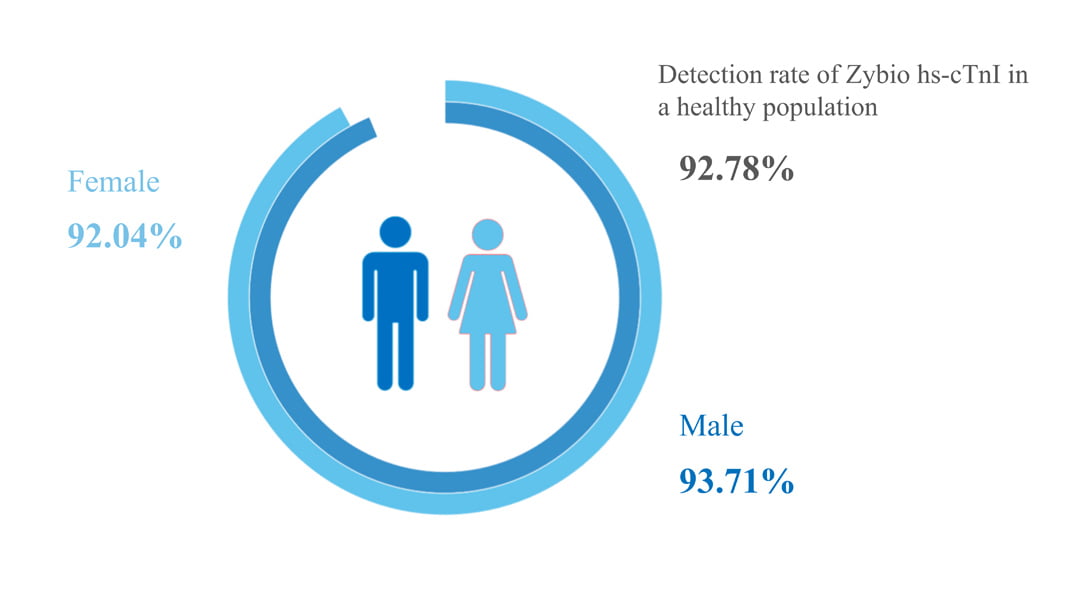
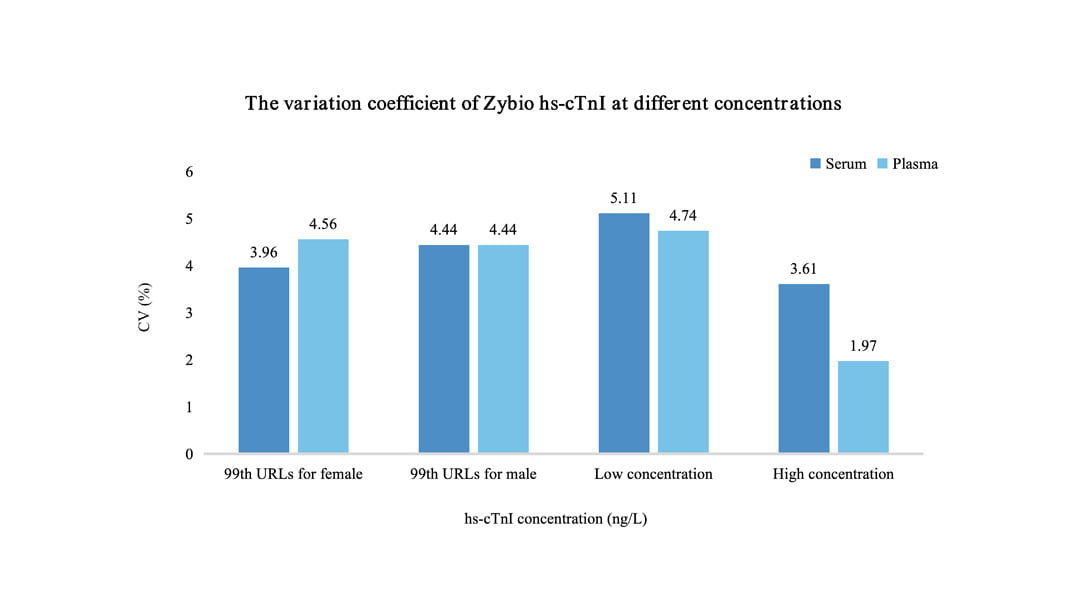
Clinically Validated, In Accordance With The Definition Of "True High-Sensitivity Troponin" by IFCC
The detection rate in the healthy population is significantly higher than the 50% required by the IFCC, achieving a sensitivity of 92.78%. The 99th percentile CV of Zybio hs-cTnI was less than 5% (IFCC requirement ≤10%). What’s more, hs-cTnI testing with serum or plasma sample features good consistency, enjoying high precision.
 |  |
Zybio has been and will always be committed to the concept of "striving for excellence" and constantly go further in technology of CLIA reagent. Meanwhile, we, with customer-centered notion in mind, will improve our services in an all-round and multi-angle manner to provide more comprehensive and efficient solutions for healthcare providers, and in this way, we safeguard patients' lives and health.
