SARS-CoV-2 Nucleic Acid Detection Kit (PCR-Fluorescent Probe Method)
May 10, 2021 Zybio News
-

December 26, 2025
DiagHub Online Academic Platform The ninth session on Microbiology Concludes Successfully – Highlights Recap
-

Nov 28, 2025
DiagHub Online Academic Platform The eighth session on Molecular Concludes Successfully – Highlights Recap
-

Nov 21, 2025
Zybio at MEDICA 2025: Comprehensive Laboratory Diagnostic Solution
On April 1st, SARS-CoV-2 Nucleic Acid Detection Kit (PCR-Fluorescent Probe Method) developed by Zybio obtained the medical device registration certificate of CFDA.
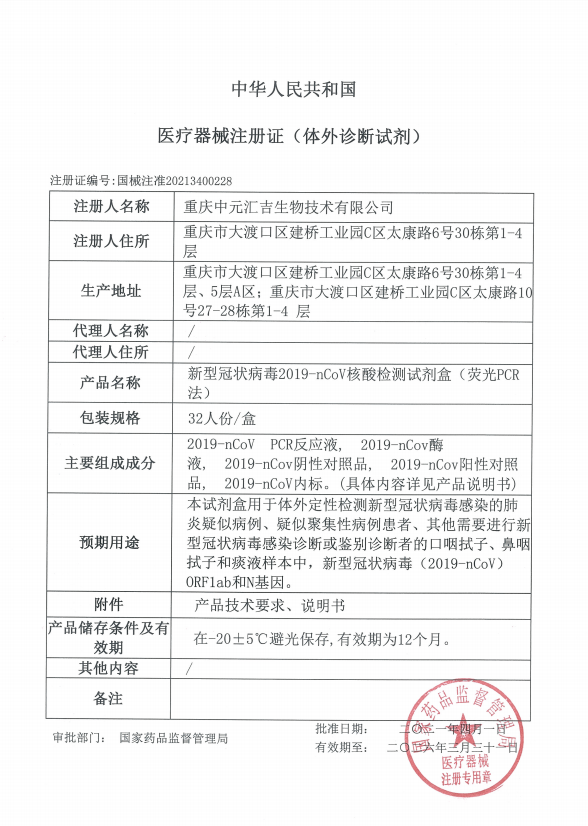
According to the Work Manual of Nucleic Acid Testing for SARS-CoV-2 in Medical Institutions released by Joint Prevention and Control Mechanism of the State Council on December 28th, 2020, it is recommended to use reagents with high sensitivity (LoD ≤500 copies/ml) and above the double target area for detection, and use the matching VTM and extraction kits recommended in the IFU.
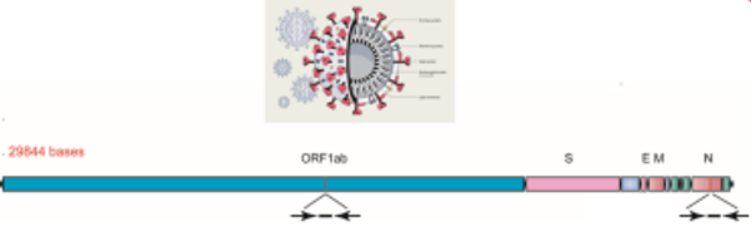
Primers and probes for SARS-CoV-2 nucleic acid detection
Furthermore, As early as March 9th, 2020, Zybio has obtained the CE certification of SARS-CoV-2 Nucleic Acid Detection Kit, providing VTM, automatic nucleic acid isolation system EXM3000, EXM6000, and nucleic acid extraction and detection kit for nearly 100 countries including ASEAN countries. Zybio's molecular products will continue to fight against the global COVID-19 pandemic and protect human health.
